The American Society of Echocardiography (ASE) has released a consensus statement on the use of ultrasound enhancing contrasting agents containing polyethylene glycol (PEG) following guidance from U.S. Food and Drug Administration (FDA).
The statement has been generated to provide all practitioners of echocardiography with information and recommendations that will benefit the safety of patients receiving ultrasound enhancing agents (UEAs). Specifically, the document provides expert opinion on the clinical impact of the recent alert from MedWatch, the U.S. Food and Drug Administration (FDA) product safety reporting system, on presumed Type I immediate hypersensitivity reactions to the polyethylene glycol (PEG) component of UEAs.
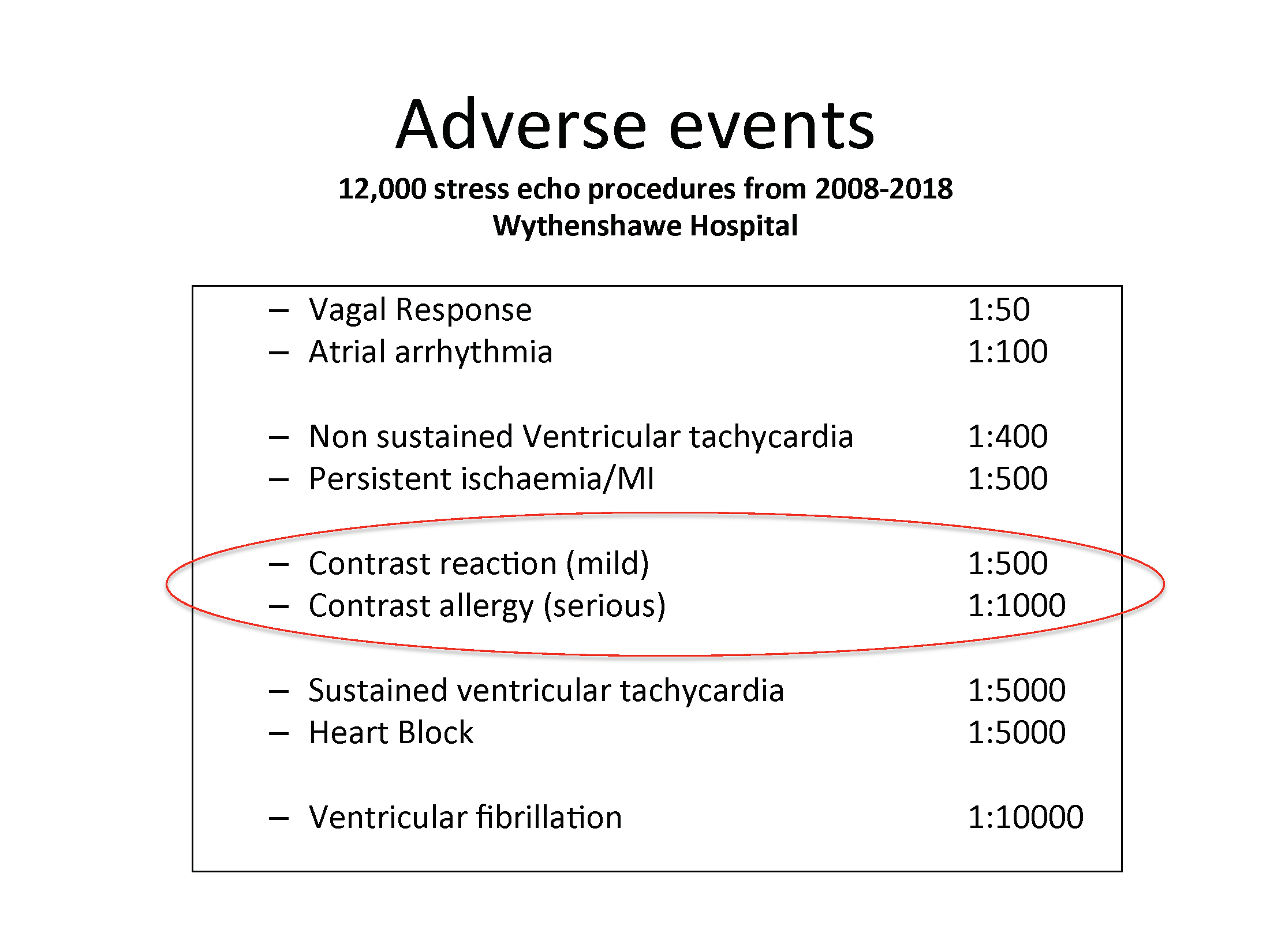
The British Society of Echocardiography (BSE) is pleased to support this guidance from the ASE as an International Alliance Partner. Dr Anita MacNab, Lead for Stress Echo Accreditation at the BSE, said "Ultrasound enhancing contrast agents have been used in stress laboratories around the UK for more than two decades and contrast allergies have probably been experienced first hand by most departments at some point. This new ASE consensus statement highlights one of the components in contrast that could cause an allergic response. The message is to ask patients specifically about a previous allergy to Polyethylene Glycol (PEG) which can be found in some laxatives and creams. Below you can see our 10 year experience of adverse events from 12,000 scans. Contrast reactions are more frequent than that reported by companies but they are usually mild. The serious reactions can be dramatic and can erode confidence in the use of such agents. However, we have to remind ourselves of the benefits these agents offer in terms of accuracy of the test results. We therefore have to consent properly, be vigilant of allergic reactions and be adequately prepared to deal with them."
The full consensus statement can be found on the ASE website below.
Read the statement
The BSE is also pleased to support the confirmatory statement from the European Association of Cardiovascular Imaging, updated for European guidance.
Read the statement